Page 3 : The Biology of Oryza sativa (Rice)
Transgenic crops, the best-analysed plants in history
The comparative approach
In 1990, a joint consultation of the Food and Agriculture Organisation of the United Nations (FAO) and the World Health Organisation (WHO) established that the comparison of a final product with one having an acceptable standard of safety provides an important element of safety assessment (WHO, 1991).
In 1993 the Organisation for Economic Co-operation and Development (OECD) further elaborated this concept and advocated the approach to safety assessment based on substantial equivalence as being the most practical approach to addressing the safety of foods and food components derived through modern biotechnology (as well as other methods of modifying a host genome, including tissue culture methods and chemical or radiation induced mutation). In 2000 the Task Force concluded in its report to the G8 that the concept of substantial equivalence will need to be kept under review (OECD, 2000).
In 2000, the Joint FAO/WHO Expert Consultation on Foods Derived from Biotechnology concluded that the safety assessment of genetically modified foods requires an integrated and stepwise, case-by-case approach, which can be aided by a structured series of questions. A comparative approach focusing on the determination of similarities and differences between the genetically modified food and its conventional counterpart aids in the identification of potential safety and nutritional issues and is considered the most appropriate strategy for the safety and nutritional assessment of genetically modified foods. The concept of substantial equivalence was developed as a practical approach to the safety assessment of genetically modified foods. It should be seen as a key step in the safety assessment process although it is not a safety assessment in itself; it does not characterise hazard, rather it is used to structure the safety assessment of a genetically modified food relative to a conventional counterpart. The consultation concluded that the application of the concept of substantial equivalence contributes to a robust safety assessment framework.
In 1996, a previous Joint FAO/WHO Expert Consultation on Biotechnology and Food Safety elaborated on compositional comparison as an important element in the determination of substantial equivalence. A comparison of critical components can be carried out at the level of the food source (ie, species) or the specific food product. Critical components are determined by identifying key nutrients, key toxicants and antinutrients for the food source in question. The comparison of critical components should be between the modified variety and non-modified comparators with an appropriate history of safe use. The data for the non-modified comparator can be the natural ranges published in the literature for commercial varieties or those measured levels in parental or other edible varieties of the species (FAO, 1996). The comparator used to detect unintended effects for all critical components should ideally be the near isogenic parental line grown under identical conditions. While the comparative approach is useful as part of the safety assessment of foods derived from plants developed using recombinant DNA technology, the approach could, in general, be applied to foods derived from new plant varieties that have been bred by other techniques.
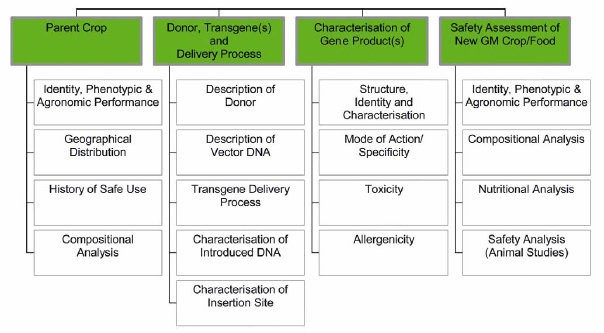
Risk assessment process: From Koenig et al. Food and Chemical Toxicology 42:1047-1088, 2004.
The role of familiarity in risk and safety assessment
The issue of scale-up also led to the important concept of familiarity, which is one key approach that has been used subsequently to address the environmental safety of transgenic plants.
The concept of familiarity is based on the fact that most genetically engineered organisms are developed from organisms whose biology is well understood, eg, crop plants. It is not a risk and safety assessment in itself (US NAS 1989). However, the concept facilitates risk and safety assessments, because to be familiar, means having enough information to be able to make a judgement of safety or risk. Familiarity can also be used to indicate appropriate management practices, including whether standard agricultural practices are adequate or whether other management practices are needed to manage the risk (OECD 1993a). Familiarity allows the risk assessor to draw on previous knowledge and experience with the introduction of plants and micro-organisms into the environment, and this indicates appropriate management practices.
As familiarity depends also on the knowledge about the environment and its interaction with introduced organisms, the risk and safety assessment in one country may not be applicable in another country. However, as field tests are performed, information will accumulate about the organisms involved, and their interactions with a number of environments.
Familiarity comes from the knowledge and experience available for conducting a risk and safety analysis prior to scale-up of any new plant line or crop cultivar in a particular environment. For plants, for example, familiarity takes account of, but need not be restricted to, knowledge and experience with:
- the crop plant, including its flowering and reproductive characteristics, ecological requirements, and past breeding experiences;
- the agricultural and surrounding environment of the trial site;
- specific trait(s) transferred to the plant line(s);
- results from previous basic research including greenhouse and small-scale field research with the new plant line or with other plant lines having the same trait;
- the scale-up of lines of the crop varieties developed by more traditional techniques of plant breeding;
- the scale-up of other plant lines developed by the same technique;
- the presence of related (and sexually compatible) plants in the surrounding natural environment, and knowledge of the potential for gene transfer between crop plant and the relative; and
- interactions between the crop, the environment and the introduced trait (OECD, 1993a).
Golden Rice has been researched thoroughly
Golden Rice has gone through many tests since it was first obtained. Among the tests performed are:
- In-depth investigation and understanding of the endosperm carotenoid biosynthetic pathway modification, which accurately explains the source of the golden colour of Golden Rice.
- Less than 10 transgenic events (from about 2000 created) were carefully selected to be able to fulfil regulatory requirements regarding the genetic structure.
- Gene expression profiling of thousands of genes was carried out, showing no unexpected changes or gross perturbances in the expression profile as compared to the parent material.
- Allergenic potential has been ruled out at the prediction level using bioinformatic analysis of transgene proteins. The report is available online at Allergenonline.
- High digestibility of the transgenic proteins in simulated gastric fluid has been demonstrated, further substantiating the claim of lack of allergenic potential.
- It has been shown that Golden Rice diverts only a minuscule amount of carbon into carotenoids, so that changes in compositional analysis are minimal.
- Various taste trials have been conducted which have not detected taste differences to the parent material.
- Tests have been conducted to determine β-carotene bioavailability and bioconversion to retinol (the most significant source of Vitamin A) by feeding deuterium-labelled Golden Rice to adults in USA as well as to a small group of children in China. Both trials were highly successful in showing that the human intestine is indeed capable of extracting β-carotene out of Golden Rice in a highly efficient manner [1,2].
The Golden Rice project is already working with regulators in some target countries. Regulators allow informed individuals to eat Golden Rice prior to commercial regulatory clearance in a country for research purposes. However, the Golden Rice project has been careful to restrict usage only to that essential to the objectives of the project. Very few people have tasted Golden Rice so far. Human studies are essential to select lines with optimal nutritional characteristics.
Animal testing is not mandated by FDA, and because animals metabolise β-carotene differently from humans, such a test would not have answered the human bioavailability and bioconversion questions which need to be answered to tell us whether Golden Rice is as good or better than β-carotene delivered in capsules or as vegetables.
[1] Tang et al (2009) Golden Rice is an effective source of vitamin A. American Journal of Clinical Nutrition 89:1776-1783
[2] Tang et al (2012) β-Carotene in Golden Rice is as good as β-carotene in oil at providing vitamin A to children. American Journal of Clinical Nutrition 96:658-664
Page 2 : Data for Golden Rice transboundary movement
Page 3 : The Biology of Oryza sativa (Rice)

